Advancing MSM Quantification in Pet Joint Health Supplements

Figure 1. 3D Structure of Methylsulfonylmethane (MSM).
Methylsulfonylmethane (MSM), chemically known as dimethyl sulfone (DMSO₂), is an organosulfur compound recognized for its role in joint health. It is commonly formulated alongside chondroitin sulfate and glucosamine, creating synergistic blends aimed at reducing inflammation, supporting cartilage integrity, and improving mobility. In addition to joint benefits, MSM may be associated with antioxidant activity and potential improvements in skin and coat health, making it a versatile ingredient in pet nutrition.
Figure 2. MSM (Left), Glucosamine (Middle), and Chondroitin Sulfate (Right). MSM is commonly formulated with glucosamine and chondroitin sulfate in pet chew supplements.
As the pet supplement market continues to expand, particularly in preventative care, accurate quantification of MSM has become essential. Manufacturers require robust analytical methods to ensure that label claims are substantiated and that formulations meet quality standards while complying with AAFCO ingredient definitions and state feed regulations.
Technical Advancements in MSM Analysis
The Eurofins Nutrition Analysis Center in Des Moines, IA has introduced a specialized analytical method for MSM in pet chew supplements, addressing the growing demand for validated testing in the pet supplement industry. This advancement supports manufacturers seeking to meet consumer expectations for preventative joint care in companion animals.
Pet chews present a complex analytical matrix due to flavoring agents, fats, binders, and varying excipients which can interfere with the extraction and chromatographic separation. This can lead to co-elution and instability with the recovery of MSM. With modifications to the USP – NF monograph for Glucosamine, Chondroitin Sulfate Sodium, and Methylsulfonylmethane Tablets – Content of Methylsulfonylmethane, this newly developed method offers several key improvements over conventional approaches:
- Enhanced Sensitivity: Capable of detecting MSM concentrations as low as 0.05%, providing confidence in trace-level quantification.
- Improved Extraction Efficiency: Optimized solubility in the extraction solution ensures higher recovery rates, reducing variability and improving reproducibility.
- Broader Applicability: Designed to accommodate diverse pet chew formulations, including complex matrices with varying excipients and flavoring agents.
In this method, diethylene glycol methyl ether (diEGME) is added as an internal standard due to chemical stability, absence from the sample matrix, and similarities in chromatographic behavior. MSM is extracted from samples and analyzed using gas chromatography with flame ionization detection (GC-FID).

Figure 3. MSM (Left) vs. DMSO (Dimethyl Sulfoxide) (Right). DMSO can be found in some human and potentially animal supplements.
Although DMSO (dimethyl sulfoxide) is not quantified with this method, it may also offer benefits in reducing pain from osteoarthritis when applied topically. Because DMSO can appear in human and pet supplements, clear chromatographic separation between DMSO and MSM is critical to avoid co-elution and ensure accurate quantification, given their distinct chemical properties. See Figure 4 for an example chromatogram.
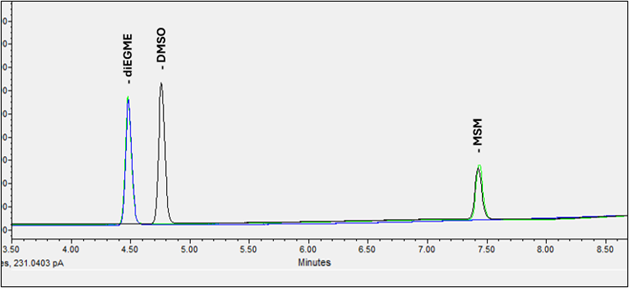
Figure 4. Chromatographic Separation and Internal Standard. Clear chromatographic separation of MSM and DMSO is critical to avoid co-elution and ensure accurate quantification, given their distinct chemical properties. diEGME is used as the internal standard.
Supporting Industry Growth
The launch of this method reflects Eurofins’ commitment to advancing analytical science in the pet supplement sector. By providing specialized nutrition analysis, Eurofins empowers brands to substantiate functional claims, mitigate non-compliance risks, and maintain competitive advantage in a rapidly evolving market.
With pet owners increasingly prioritizing preventative health solutions, validated testing methods such as Eurofins’ MSM analysis are critical for ensuring product integrity and supporting the credibility of joint health supplements.
Meet the author:
Emily McManus, Associate Method Development Scientist, Eurofins Nutritional Analysis Center, Des Moines, IA
Emily McManus is one of the scientists on the Method Development Team at Eurofins Nutrition Analysis Center (ENAC) in Des Moines, IA. Emily has spent over 8 years at Eurofins and began as a laboratory technician in the Vitamins department before transitioning as a scientist on the Method Development team in 2022. Emily’s expertise at ENAC has been with water soluble vitamins before expanding into new areas of analysis. Emily was a leading method development scientist for the Methylsulfonylmethane (MSM) by GC-FID analysis study in Des Moines.



