Strengthening Ingredient Integrity: How Robust Testing Protects Your Pet Food Supply Chain

In the competitive and highly regulated pet food industry, ingredient authenticity is more than a quality metric; it's a cornerstone of consumer trust and brand integrity. As demand for functional ingredients grows, so does the risk of adulteration. Manufacturers must be equipped with advanced testing strategies to safeguard their supply chains and ensure label claims are accurate.
The Hidden Risk of Adulteration
Adulteration involves mixing or cutting active ingredients with similar compounds to reduce production costs. While some adulterants may not pose immediate health risks, they can lead to:
- Fraudulent label claims
- Regulatory penalties
- Loss of consumer trust
- Potential harm to pets, as seen in past incidents like melamine contamination
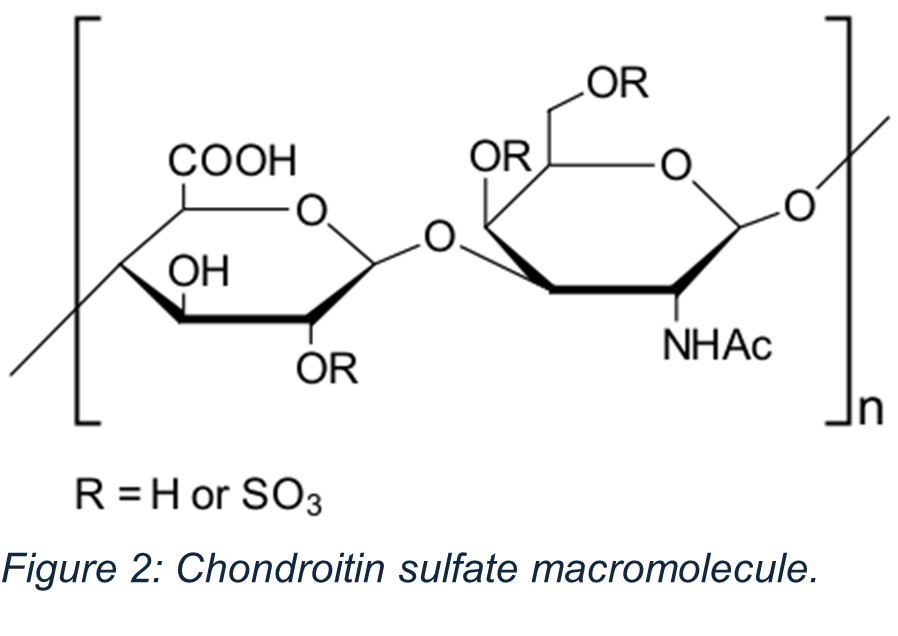
One ingredient that may be particularly vulnerable to adulteration is chondroitin sulfate (CS), a glycosaminoglycan found in cartilage and widely used in joint health supplements for pets. Due to its popularity and cost, CS has been found to be adulterated with compounds like glucuronic acid and carrageenan, which mimic CS in basic turbidity titration tests due to their similar negative charge.

Why Enhanced Testing Methods Matter
Traditional methods such as turbidity titration have long played a valuable role in estimating chondroitin sulfate (CS) content. These approaches are well-established, cost-effective, and suitable for many raw material applications. However, when it comes to complex matrices, like finished pet food products or multi-ingredient pet health supplements, these methods may face limitations in specificity and accuracy.
In such cases, advanced analytical techniques are essential to confidently distinguish authentic CS from structurally similar compounds. Ingredients like glucuronic acid and
carrageenan can mimic CS in basic titration assays due to their similar charge properties, potentially leading to overestimation or misidentification.
To address these challenges, Eurofins Nutrition Analysis Center (ENAC) has developed and validated specialized methods tailored for complex formulations, including:
- UPLC-FLR with Chondroitinase AC I: This method enzymatically breaks down CS into unique disaccharide profiles, which are then quantified through liquid chromatography. It provides a highly specific fingerprint of CS content, even in multi-component products.
- Glucosamine by LC-MS: This technique detects glucosamine and glucosamine-related impurities that help identify degradation or adulteration, offering an additional layer of verification.

These enhanced methods are designed to complement traditional testing, not replace it. By layering both approaches, manufacturers can build a more complete picture of ingredient integrity ensuring that label claims are accurate, regulatory standards are met, and consumer trust is upheld.

In short, robust testing strategies are about choosing the right tool for the right matrix. For complex pet nutrition products, advanced methods capable of cleaning and targeting offer the clarity and confidence needed to navigate today’s quality and compliance landscape.
Beyond Compliance: Building a Resilient Supply Chain Through Robust Testing
In today’s pet food and supplement industry, regulatory compliance is just the starting point. True resilience comes from proactive quality assurance, where manufacturers use advanced analytical methods not only to meet standards—but to exceed them. Robust testing is a strategic investment that fortifies every link in the supply chain.
Here’s how:
Ensure Product Consistency
Advanced testing methods—especially those tailored for complex matrices—help manufacturers maintain consistent ingredient quality across batches. This reduces variability, supports formulation integrity, and ensures that every product performs as expected.
Validate Label Claims
Accurate quantification of active ingredients like chondroitin sulfate is essential for truthful labeling. Techniques such as UPLC-FLR and LC-MS provide the specificity needed to confirm that what's on the label matches what's in the product, protecting brands from misrepresentation and regulatory scrutiny.
Minimize Risk of Recalls or Litigation
Adulterated or mislabeled ingredients can lead to costly recalls, legal action, and reputational damage. By detecting impurities and verifying authenticity before products reach the market, robust testing acts as a safeguard against these risks.
Strengthen Consumer Loyalty
Consumers expect transparency and safety. When manufacturers consistently deliver high-quality, accurately labeled products, they build trust. That trust translates into brand loyalty, repeat purchases, and positive word-of-mouth.
Support Supply Chain Transparency
Robust testing also enables better supplier accountability. With precise data on ingredient purity and composition, manufacturers can confidently evaluate vendors, negotiate contracts, and trace quality issues back to their source.
In short, robust testing isn’t just a lab function, it’s a business strategy. It empowers manufacturers to deliver safe, effective products while building a supply chain that’s agile, transparent, and trusted.
Ready to Verify Your Ingredient?
Whether you're formulating a new pet health supplement or auditing your current supply chain, our experts at Eurofins are here to help. Reach out today to learn how our testing solutions can support your quality assurance goals.
Meet the author:
Dr. Luis A. Camacho III is a method development scientist within the Vitamins and Special Analysis business unit of ENAC. Luis is a leading method development scientist for chondroitin sulfate analysis and assisted with glucosamine method development. His approach to method development examines what chemical properties can be used for easier and quality analysis during extraction, isolation, and UPLC analysis.
Luis earned his PhD at Iowa State University in physical organic chemistry. He specialized in solid-phase peptide chemistry and used UPLC and HPLC instrumentation to analyze his final peptide products. His work was instrumental for developing thioamide protecting groups and installation of amidine functionalities along the backbone of single peptide chains.



